Recent News
- Simple and portable spectrochemical probe for rapid detection of chlorides ions in water September 8, 2021
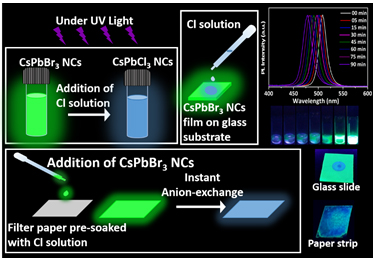 Dr Nimai Mishra, Assistant Professor, Department of Chemistry, SRM University-AP, Andhra Pradesh, along with his research group comprising of students pursuing PhD under him, Ms V.G.Vasavi Dutt and Mr Syed Akhil has published a research article titled Cesium Lead Bromide Perovskite Nanocrystals as a Simple and Portable Spectrochemical Probe for Rapid Detection of Chlorides in the Journal ChemistrySelect (Publisher: Wiley-VCH on behalf of Chemistry Europe, Impact Factor-2.2).
Dr Nimai Mishra, Assistant Professor, Department of Chemistry, SRM University-AP, Andhra Pradesh, along with his research group comprising of students pursuing PhD under him, Ms V.G.Vasavi Dutt and Mr Syed Akhil has published a research article titled Cesium Lead Bromide Perovskite Nanocrystals as a Simple and Portable Spectrochemical Probe for Rapid Detection of Chlorides in the Journal ChemistrySelect (Publisher: Wiley-VCH on behalf of Chemistry Europe, Impact Factor-2.2).Chloride anions are widely abundant in water and when they combine with calcium, potassium, and magnesium, they form chloride salts. However, the higher concentrations badly affect the environment by causing severe dehydration and even plant death. High concentrations of sodium chloride exhibit the potential of corrosive damage thereby releasing toxic metals from plumbing fixtures. Hence, there is a need to monitor the concentration levels of chloride salts in water. Several techniques like titration, spectrophotometry, ion chromatography, electrochemistry, etc have been reported to date. Despite the high accuracy and precision of these techniques, they involve expensive instrumentation and is out of reach from on-site detection. Hence, it is necessary to look for simple, portable, and cost-effective strategies for the detection of chlorides in the water.
In this article, Dr Mishra’s research group demonstrated that the wide spectral tunability of CsPbBr3 perovskite nanocrystals (NCs) via instantaneous and facile anion exchange, make them a suitable candidate for chloride detection. Rapid anion-exchange processes between CsPbBr3 perovskite NCs and different chloride solutions were carried out in ambient conditions. The resultant anion-exchanged CsPbCl3-xBrx NCs preserved the structural properties and exhibited a remarkable blue shift in photoluminescence spectra. This forms a basis for the detection of chloride ions in water. This has been applied with the limit of detection up to 100 µM. The detection strategies were not only limited to the direct addition of chloride solutions to NCs, but they also showed a visual colour change under UV light when the chloride solution is drop-casted on CsPbBr3 films that are deposited on glass substrates. Furthermore, the detection strategy is established by drop-casting CsPbBr3 NCs onto paper strips that are pre-soaked in chloride solutions. A considerable blue shift in fluorimetry proves them to be an excellent sensing medium as practical spectrochemical probes for on-site detection of chlorides. Based on this, a colour chart and selectivity chart to access the presence of chlorides and their concentration is also demonstrated.
Continue reading → - Prof U Ramamurty, renowned researcher from NTU Singapore, visits SRM University-AP September 7, 2021
 An interactive session between Prof U Ramamurty, President Chair Professor, School of Mechanical & Aerospace Engineering at Nanyang Technological University (NTU), Singapore, and the faculty members of SRM University – AP, Andhra-Pradesh was held on Monday.
An interactive session between Prof U Ramamurty, President Chair Professor, School of Mechanical & Aerospace Engineering at Nanyang Technological University (NTU), Singapore, and the faculty members of SRM University – AP, Andhra-Pradesh was held on Monday.During the discussion, Prof Ramamurty emphasized the importance of research collaboration between faculty members from different research areas and about utilizing expertise to achieve significant scientific output.
Dr Pardhasaradhi Maram from the Department of Chemistry, Dr Sabyasachi Mukhopadhyay from the Department of Physics, and Prof G S Vinod Kumar from the Department of Mechanical Engineering presented their detailed research areas that focus on storage devices, catalysts for value-added products, energy and sensing devices, novel metallic materials, additive manufacturing of metals and Bio-implants, and industry collaborative research work.
Prof Ramamurty said that he is glad to see that productive science is being done at SRM University-AP. “Given that the University has started only 4 years ago and been functioning amidst a pandemic for more than one and a half years, the progress in research is significant and very impressive. Interdisciplinary efforts between various departments in the University will give effective results”, he added.
Prof D Narayana Rao, Pro-Vice-Chancellor, SRM University – AP expressed his interest in establishing NTU – SRM joint Centre for Advanced Research in functional and structural materials at SRM University campus to Prof Ramamurty. The centre that Prof Rao envisions will provide an opportunity to synergize the expertise and resources of NTU, Singapore, and SRM University – AP to carry out front-line research in the areas of novel materials, self-healing materials and also additive manufacturing (3D Printing of metals and bio-implants).
Continue reading → - Advances of surface-enhanced Raman and IR spectroscopies August 16, 2021
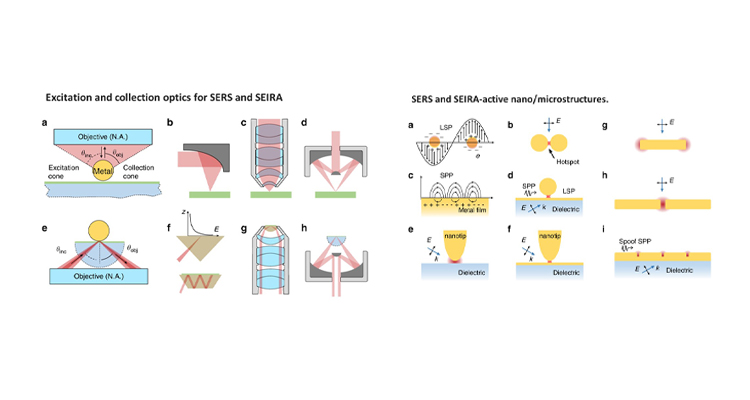 Dr Rajapandiyan Paneerselvam from the Department of Chemistry has published a paper titled “Advances of surface-enhanced Raman and IR spectroscopies: from nano/microstructures to macro-optical design” in the journal Light: Science & Applications, Volume 10, Article number: 161 (2021) having an Impact factor of 17.7.
Dr Rajapandiyan Paneerselvam from the Department of Chemistry has published a paper titled “Advances of surface-enhanced Raman and IR spectroscopies: from nano/microstructures to macro-optical design” in the journal Light: Science & Applications, Volume 10, Article number: 161 (2021) having an Impact factor of 17.7.Raman and infrared (IR) spectroscopy are powerful analytical techniques, which are widely used for a variety of applications including food analysis, environmental analysis, chemical, and biomolecule analysis. This review article presents some latest advancements in vibrational spectroscopic techniques, and further developments in this field are given with emphasis on emerging techniques and methodologies.
This article has been published with Prof Zhong-Qun Tian’s group, State Key Laboratory of Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005, China.
Furthermore, Dr Rajapandiyan’s research group will focus on the development of plasmonic nanostructures for surface-enhanced Raman spectroscopy and its applications in food science, spectroelectrochemistry, and microfluidics in the future.
Read the full paper here: https://doi.org/10.1038/s41377-021-00599-2
Continue reading → - Dr Nimai Mishra’s research group studies new surface capping ligands August 2, 2021
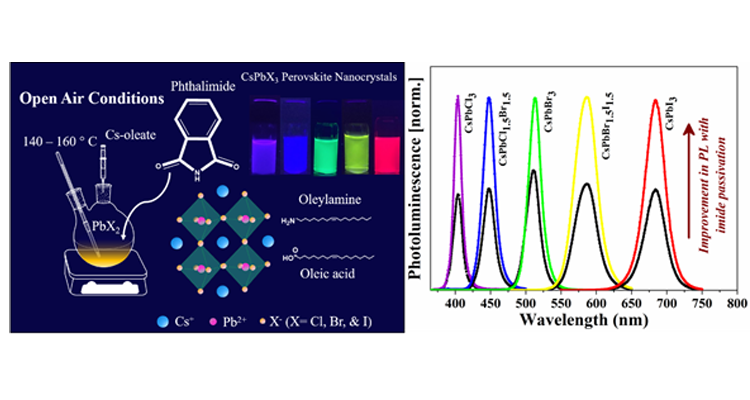 Dr Nimai Mishra, Assistant Professor, Department of Chemistry, SRM University-AP, Andhra Pradesh, along with his research group pursuing PhD under him-Ms V.G.Vasavi Dutt and Mr Syed Akhil- have published a research article titled “Enhancement of Photoluminescence and Stability of CsPbX3 (X= Cl, Br, and I) Perovskite Nanocrystals with Phthalimide Passivation” in the Journal “Nanoscale” (The Royal Society of Chemistry, Impact Factor-7.8).
Dr Nimai Mishra, Assistant Professor, Department of Chemistry, SRM University-AP, Andhra Pradesh, along with his research group pursuing PhD under him-Ms V.G.Vasavi Dutt and Mr Syed Akhil- have published a research article titled “Enhancement of Photoluminescence and Stability of CsPbX3 (X= Cl, Br, and I) Perovskite Nanocrystals with Phthalimide Passivation” in the Journal “Nanoscale” (The Royal Society of Chemistry, Impact Factor-7.8).Caesium lead halide perovskite nanocrystals (CsPbX3 NCs) have been the flourishing area of research in the field of photovoltaic and optoelectronic applications because of their excellent optical and electronic properties. However, they suffer from low stability and deterioration of photoluminescence (PL) properties post-synthesis. One of the ways to minimize the surface defects in the surface treatment with suitable ligands is to achieve the NCs with superior PL properties for light-emitting applications.
In this article, Dr Mishra’s research group demonstrates that incorporating an additional ligand can further enhance the optical properties and stability of NCs. Here, we introduced phthalimide as a new surface passivation ligand into the oleic acid/oleylamine system in situ to get near-unity photoluminescence quantum yield (PLQY) of CsPbBr3 and CsPbI3 perovskite NCs. We observed, phthalimide passivation dramatically improves the stability of CsPbCl3, CsPbBr3, and CsPbI3 NCs under ambient light and UV light. The PL intensity is recorded for one year which showed a dramatic improvement for CsPbBr3 NCs. Nearly 11% of PL can be retained even after one year for phthalimide passivated samples, on the other hand, the PL of as-synthesized NCs completely diminishes in four months. CsPbCl3 NCs exhibit 3 times higher PL with phthalimide and retain 12% PL intensity even after two months while PL of as-synthesized NCs completely diminishes by then. Under continuous UV light illumination, the PL intensity of phthalimide passivated NCs is well preserved while the as-synthesized NCs exhibit negligible PL emission in 2 days. About 40% and 25% of initial PL is preserved for CsPbBr3 and CsPbCl3 NCs in the presence of phthalimide. CsPbI3 NCs with phthalimide exhibit PL even after 2 days while the PL is rapidly declined for as-synthesized NCs in the first 10 hours. The presence of phthalimide in CsPbI3 NCs could maintain stability even after a week while the as-synthesized NCs under transition to non-luminescent phase within 4 days.
Furthermore, blue, green, yellow, and red-emitting diodes by using CsPbCl1.5Br1.5, CsPbBr3, CsPbBr1.5I1.5, CsPbI3 NCs respectively are fabricated by drop-casting NCs onto blue LED lights which show the great potential of the use of these phthalimide passivated NCs in the field of display and light technologies.
Read the full paper here: https://pubs.rsc.org/en/content/articlelanding/2021/nr/d1nr03916d
Continue reading →











