- Embarking on a new journey with pride June 29, 2022
 Final year BSc (Hons) Integrative Biology student Mr Omkar Mani Kanteswar has received an admission offer for the Master of Science in Biology at the University of Memphis (UofM), USA. Omkar’s happiness has no bounds as he really worked hard to get into his dream university.
Final year BSc (Hons) Integrative Biology student Mr Omkar Mani Kanteswar has received an admission offer for the Master of Science in Biology at the University of Memphis (UofM), USA. Omkar’s happiness has no bounds as he really worked hard to get into his dream university.The University of Memphis is a public research university in Memphis, Tennessee. Since Omkar is from a Bsc Biology background, he wanted to continue in the same research field. While shortlisting the universities, he found this research-oriented college. He passed the Duolingo test and IELTS to get into this college.
The Master of Science (MS) in Biology is a research-centred degree programme with an intensive core and elective curriculum. Omkar opted for this course owing to the fact that he wanted to continue his research in the field of Biology. His message for the junior batches is to be confident and independent to make their own decisions and not run with the herd. He urges them to focus on holistic development and extracurricular activities. He is also ready to provide support to them if they need any.
He extended his gratitude to the faculty members who helped him a lot in the process by giving him confidence. He was glad to have them because they consistently and positively helped him in his academics and choosing various colleges. Faculty from the Department of Biological Sciences- Prof Jayaseelan Murugaiyan (HOD), Assistant Professor Sutharsan Govindarajan, and Prof Imran Pancha hold significant positions in Omkar’s career venture.
Admission to a reputed institute means a sense of pride, the joy of knowing you would study the best things with the best ones. Omkar believes that this is just the beginning, and he is yet to do a lot more things with his precious life.
Continue reading → - Pave your pathway to Ivy League Universities June 29, 2022

Have you ever been nervous at the thought of writing competitive exams? Have you ever tried tricks to get the better of such exams? It is very natural that students hold fear and dislike towards such tests as they were conditioned to believe that their future hangs at the mercy of an examination. Preparing for such standardised academic tests is a complicated process as one barely knows what to learn and where to start. But the more critical question is how to learn. A clear picture of how to learn and give their best shot at such tests will help smoothen their pathway.
The Office of International Relations and Higher Studies has scheduled a webinar on ‘Abroad Education and the Road to Ivy League Universities’ with Mr J V Murty as the keynote speaker. He will walk students through the learning process of different types of standardised tests to grab a great score and ease their pathway towards overseas education.
Date: June 29, 2022
Time: 11.00am to 3.45pm
About the Speaker
Mr J V Murty is one of the most revered educators in the field of training for competitive examinations and abroad education consulting in the country. He specialises in preparing students to ivy league universities in the USA and IIMs/ISB in India.
He is a gold medalist from NIT Rourkela. He worked for the Visakhapatnam Steel Plant and Siemens in West Germany. He served as Director for T.I.M.E for more than 20 years. He has also trained and sent more than 3000 students to different IIMs and prestigious Business Schools during this time.
He served as Vice-chairman for Woxsen University and is currently working as CEO at Ashoka School of Business.
He gave CAT 17 times and scored 100% 4 times. He also holds a 339 out of 340 in GRE and a 9 on 9 in IELTS.
Join the webinar with Mr J V Murty, the master trainer, and chart your path to the Ivy League Universities!
- Dr Siddhant Dash June 27, 2022
- Paper accepted in the prestigious conference to be held in Caneda June 27, 2022
The research paper, An under complete autoencoder for denoising computational 3D sectional images from the Department of Electronics and Communication Engineering has been accepted in a prestigious conference called Imaging and Applied Optics Congress to be held in Vancouver, Canada 2022. Assistant Professors; Dr Sunil Chinnadurai, Dr Karthikeyan Elumalai, Dr Inbarasan Muiraj, and the PhD students; Ms Vineela Chandra Dodda and Ms Lakshmi Kuruguntla are the authors who contributed to composing the paper.
Abstract
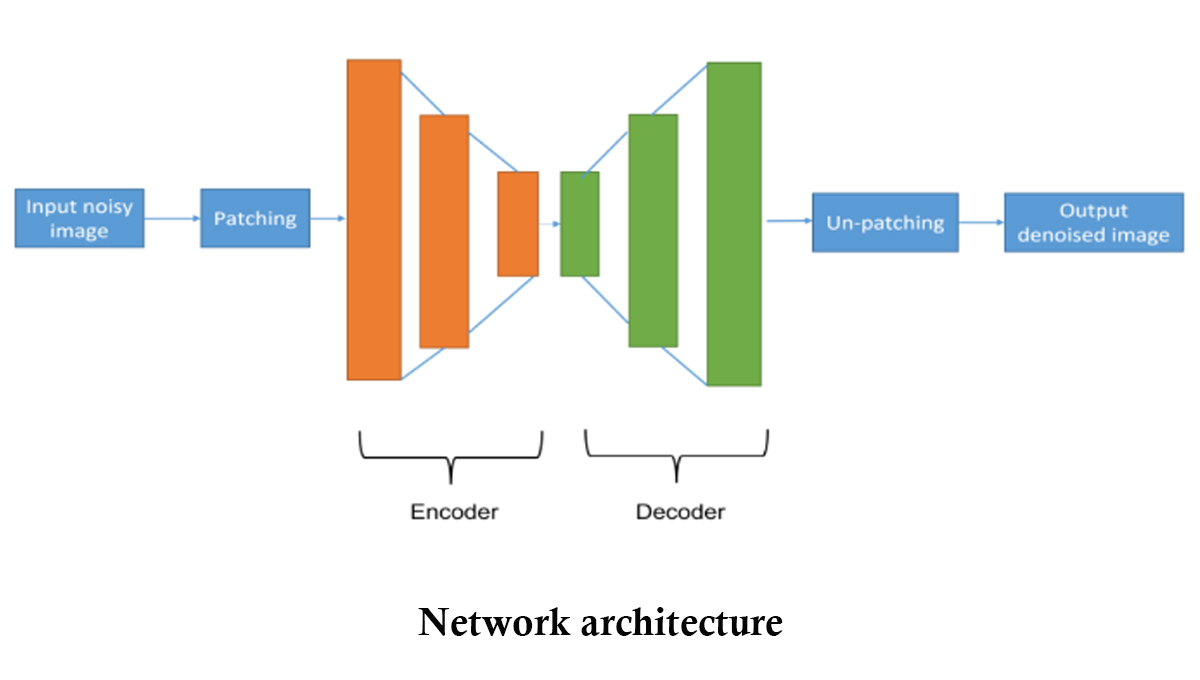 This paper proposes to use a deep-stacked under complete autoencoder to denoise the noisy 3D integral (sectional) images with a patch-based approach. In this process, the noisy input 3D sectional image is divided into multiple patches, which are then used to train the neural network. By using the patch-based approach, the time required to prepare the labeled training data is greatly reduced. Results demonstrate the feasibility of our proposed model in terms of the peak-signal-to-noise ratio.
This paper proposes to use a deep-stacked under complete autoencoder to denoise the noisy 3D integral (sectional) images with a patch-based approach. In this process, the noisy input 3D sectional image is divided into multiple patches, which are then used to train the neural network. By using the patch-based approach, the time required to prepare the labeled training data is greatly reduced. Results demonstrate the feasibility of our proposed model in terms of the peak-signal-to-noise ratio.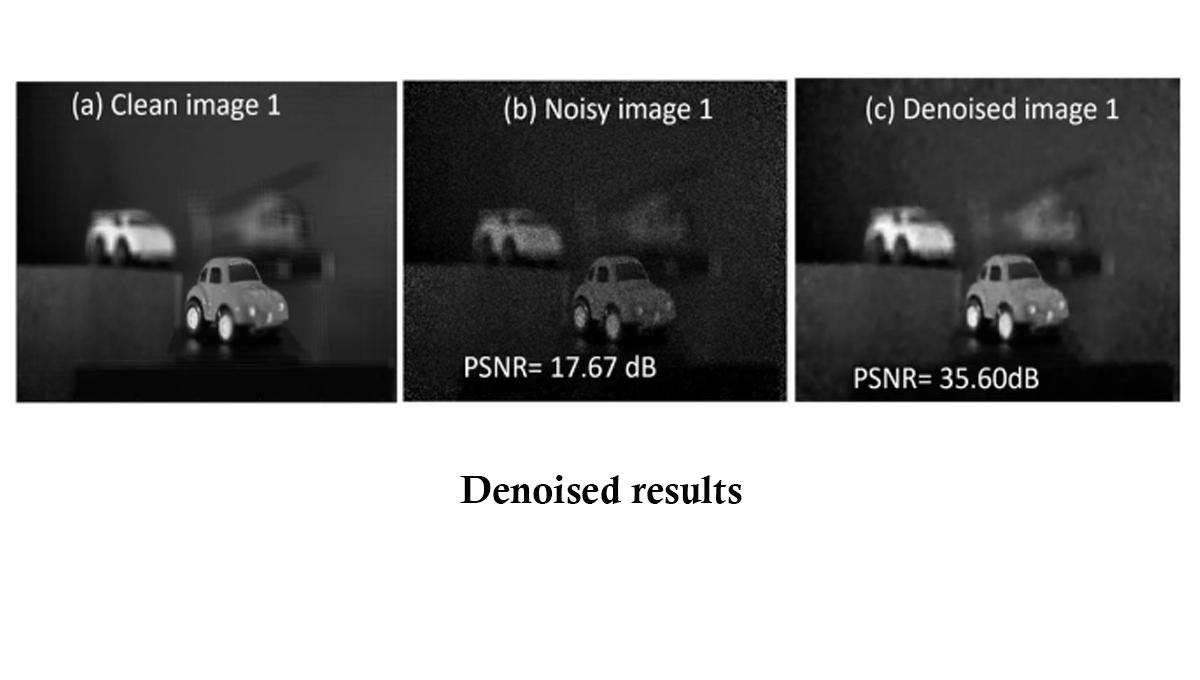 Explanation of the research
Explanation of the researchDenoising is one of the preliminary processes in image processing that removes noise from an image of interest and restores a clean image. The noise which was generated during the image acquisition process is attenuated using deep learning techniques. The denoised image is further used in various tasks of image processing.
In any image acquisition system, noise is inevitable and needs to be attenuated before further processing for qualitative results. The medical field is an example of this (images acquired through CT, MRI, PET, etc.). The researchers further investigate various techniques in deep learning to improve the denoising performance along with the applicability of deep learning in various tasks such as object recognition etc.
Continue reading → - A new aqueous electrolyte to enhance the yield of Ammonia June 25, 2022
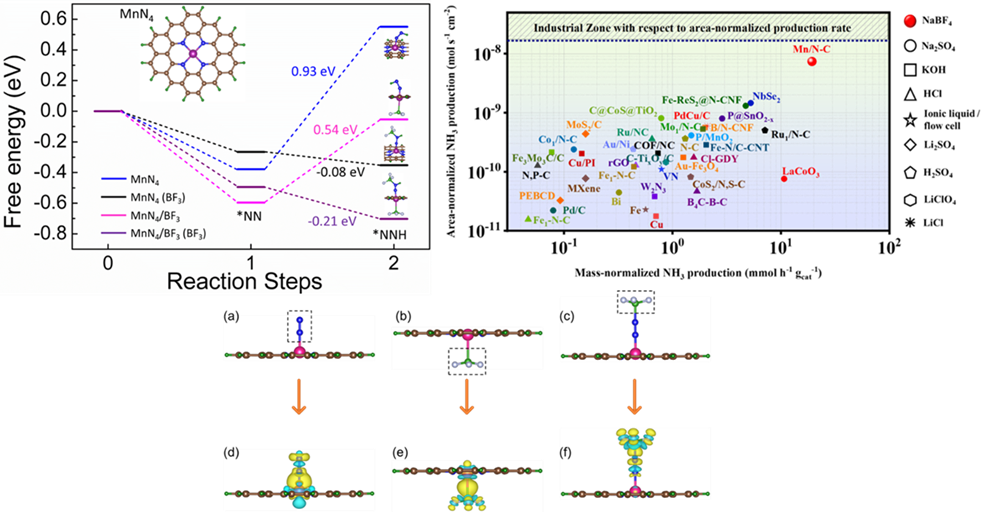
The Department of Physics is proud to announce that Prof Ranjit Thapa and his PhD scholar Mr Samadhan Kapse have published an article titled “Lewis acid-dominated aqueous electrolyte acting as co-catalyst and overcoming N2 activation issues on catalyst surface” in the most prestigious and highly cited multidisciplinary research journal, ‘Proceedings of the National Academy of Sciences’ (PNAS), having an Impact Factor of 11.2. The research was done in collaboration with Ms Ashmita Biswas, Mr Bikram Ghosh, and Dr. Ramendra Sundar Dey from the Institute of Nano Science and Technology (INST), Punjab.
Abstract of the Research
The growing demands for ammonia in agriculture and transportation fuel stimulate researchers to develop sustainable electrochemical methods to synthesize ammonia ambiently, to get past the energy-intensive Haber Bosch process. But the conventionally used aqueous electrolytes limit N2 solubility leading to insufficient reactant molecules in the vicinity of the catalyst during electrochemical nitrogen reduction reaction (NRR). This hampers the yield and production rate of ammonia, irrespective of how efficient the catalyst is. Herein we introduce a new aqueous electrolyte (NaBF4), which not only acts as an N2-carrier in the medium but also works as a full-fledged “co-catalyst” along with our active material MnN4 to deliver high yield of NH3 (328.59 μg h-1 mgcat-1) at 0.0 V vs RHE. BF3-induced charge polarization shifts the metal d-band center of MnN4 unit close to the Fermi level, inviting N2 adsorption facilely. The Lewis acidity of the free BF3 molecules further propagates their importance in polarizing the N≡N bond of the adsorbed N2 and its first protonation. This push-pull electronic interaction has been confirmed from the change in d-band center values of MnN4 site as well as charge density distribution over our active model units, which turned out to be effective enough to lower the energy barrier of the potential determining steps of NRR. Resultantly, a high production rate of NH3 (7.37 × 10-9 mol s-1 cm-2) was achieved, approaching the industrial scale where the source of NH3 was thoroughly studied and confirmed to be chiefly from the electrochemical reduction of the purged N2 gas.
A Brief Summary of the Research
The widely highlighted problem of NRR is that the competitive HER is most likely worked upon with several catalyst development and electrolyte modifications, while the N2 solubility and activation issues in the aqueous medium are generally neglected. This work justifies our aim to contribute towards this troublemaker by using NaBF4 as a working electrolyte, which served as a “full-packaged co-catalyst” along with MnN4, reinforcing the NRR kinetics at the cost of low overpotential. The Lewis-acidic nature of BF3 induced adduct formation with the N2 molecules acted as a carrier of N2 gas into the medium in vicinity of the electrocatalyst. Simultaneously, the charge polarization over MnN4 active site due to BF3 delocalized the metal d-band centre, which triggered N2 adsorption on the catalyst site. Under this condition, free BF3 form the medium interacted with the adsorbed N2 and brought about the facile polarization of the N≡N bond and its first protonation at a much lower energy barrier. This push-pull charge transfer effect enormously helped to overcome the potential determining steps and this BF3 mediated NRR resulted in a huge production rate of NH3, which could be compared to that of industrial scale, which was not achieved so far with any aqueous or ionic liquid electrolytes. In short, this kind of user-friendly aqueous electrolyte is being investigated for the first time for NRR. Since BF3 displayed tremendous potential in triggering the kinetics of NRR, this new finding may encourage researchers to work more on aqueous electrolyte designing towards an even improved NRR performance of the electrocatalysts. Not only that, electrocatalysts could also be functionalized with BF3 derivatives, which could be one entirely new route of study in the field of NRR.
Social Implications
Ammonia is considered as the most abundant and widely used synthetic fertilizer in the world. The sole mean of large-scale ammonia production relies on the century-old Haber-Bosch process, which takes in more energy than it can produce, while the electrochemical nitrogen reduction reaction (NRR) offers a carbon-free and sustainable way of ammonia synthesis. However, electrochemical NH3 synthesis is often arrested by a few factors such as NH3 detection, contaminations from source gases, nitrogen-containing chemicals and the presence of labile nitrogen in the catalysts. In the recent past, several protocols have been proposed to correct the fallacious results. Recently, Choi et el. have concluded that it is difficult to believe from the too-low yield rate of NH3 that the reduction of N2 has actually occurred in the aqueous medium. It is noteworthy that the electrolyte plays a crucial role and offers a suitable environment for any electrochemical reactions to occur. However, the issue with the solubility of N2 in conventional aqueous electrolytes is a real troublemaker to achieve a high yield and production rate of NH3 during electrochemical synthesis. Therefore, it is necessary to solve the most important issue i.e., to solvate a promising concentration of N2 molecules into the electrolyte such that it becomes accessible to the catalyst surface for its subsequent reduction.

