A new aqueous electrolyte to enhance the yield of Ammonia
The Department of Physics is proud to announce that Prof Ranjit Thapa and his PhD scholar Mr Samadhan Kapse have published an article titled “Lewis acid-dominated aqueous electrolyte acting as co-catalyst and overcoming N2 activation issues on catalyst surface” in the most prestigious and highly cited multidisciplinary research journal, ‘Proceedings of the National Academy of Sciences’ (PNAS), having an Impact Factor of 11.2. The research was done in collaboration with Ms Ashmita Biswas, Mr Bikram Ghosh, and Dr. Ramendra Sundar Dey from the Institute of Nano Science and Technology (INST), Punjab.
Abstract of the Research
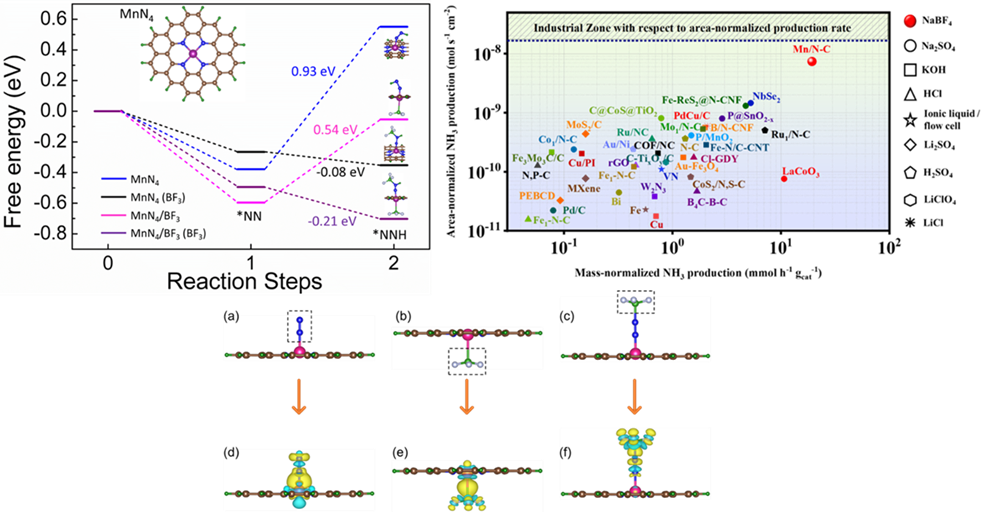
The growing demands for ammonia in agriculture and transportation fuel stimulate researchers to develop sustainable electrochemical methods to synthesize ammonia ambiently, to get past the energy-intensive Haber Bosch process. But the conventionally used aqueous electrolytes limit N2 solubility leading to insufficient reactant molecules in the vicinity of the catalyst during electrochemical nitrogen reduction reaction (NRR). This hampers the yield and production rate of ammonia, irrespective of how efficient the catalyst is. Herein we introduce a new aqueous electrolyte (NaBF4), which not only acts as an N2-carrier in the medium but also works as a full-fledged “co-catalyst” along with our active material MnN4 to deliver high yield of NH3 (328.59 μg h-1 mgcat-1) at 0.0 V vs RHE. BF3-induced charge polarization shifts the metal d-band center of MnN4 unit close to the Fermi level, inviting N2 adsorption facilely. The Lewis acidity of the free BF3 molecules further propagates their importance in polarizing the N≡N bond of the adsorbed N2 and its first protonation. This push-pull electronic interaction has been confirmed from the change in d-band center values of MnN4 site as well as charge density distribution over our active model units, which turned out to be effective enough to lower the energy barrier of the potential determining steps of NRR. Resultantly, a high production rate of NH3 (7.37 × 10-9 mol s-1 cm-2) was achieved, approaching the industrial scale where the source of NH3 was thoroughly studied and confirmed to be chiefly from the electrochemical reduction of the purged N2 gas.
A Brief Summary of the Research
The widely highlighted problem of NRR is that the competitive HER is most likely worked upon with several catalyst development and electrolyte modifications, while the N2 solubility and activation issues in the aqueous medium are generally neglected. This work justifies our aim to contribute towards this troublemaker by using NaBF4 as a working electrolyte, which served as a “full-packaged co-catalyst” along with MnN4, reinforcing the NRR kinetics at the cost of low overpotential. The Lewis-acidic nature of BF3 induced adduct formation with the N2 molecules acted as a carrier of N2 gas into the medium in vicinity of the electrocatalyst. Simultaneously, the charge polarization over MnN4 active site due to BF3 delocalized the metal d-band centre, which triggered N2 adsorption on the catalyst site. Under this condition, free BF3 form the medium interacted with the adsorbed N2 and brought about the facile polarization of the N≡N bond and its first protonation at a much lower energy barrier. This push-pull charge transfer effect enormously helped to overcome the potential determining steps and this BF3 mediated NRR resulted in a huge production rate of NH3, which could be compared to that of industrial scale, which was not achieved so far with any aqueous or ionic liquid electrolytes. In short, this kind of user-friendly aqueous electrolyte is being investigated for the first time for NRR. Since BF3 displayed tremendous potential in triggering the kinetics of NRR, this new finding may encourage researchers to work more on aqueous electrolyte designing towards an even improved NRR performance of the electrocatalysts. Not only that, electrocatalysts could also be functionalized with BF3 derivatives, which could be one entirely new route of study in the field of NRR.
Social Implications
Ammonia is considered as the most abundant and widely used synthetic fertilizer in the world. The sole mean of large-scale ammonia production relies on the century-old Haber-Bosch process, which takes in more energy than it can produce, while the electrochemical nitrogen reduction reaction (NRR) offers a carbon-free and sustainable way of ammonia synthesis. However, electrochemical NH3 synthesis is often arrested by a few factors such as NH3 detection, contaminations from source gases, nitrogen-containing chemicals and the presence of labile nitrogen in the catalysts. In the recent past, several protocols have been proposed to correct the fallacious results. Recently, Choi et el. have concluded that it is difficult to believe from the too-low yield rate of NH3 that the reduction of N2 has actually occurred in the aqueous medium. It is noteworthy that the electrolyte plays a crucial role and offers a suitable environment for any electrochemical reactions to occur. However, the issue with the solubility of N2 in conventional aqueous electrolytes is a real troublemaker to achieve a high yield and production rate of NH3 during electrochemical synthesis. Therefore, it is necessary to solve the most important issue i.e., to solvate a promising concentration of N2 molecules into the electrolyte such that it becomes accessible to the catalyst surface for its subsequent reduction.
- Published in Departmental News, News, Physics News, Research News
100% Erasmus Mundus Scholarship and MS opportunities in Europe
 SRM University-AP has numerous success stories and student accomplishments to share with the world. What makes the story of Bennet Benny different is the magnitude of his winning and the miles he has crossed after setting foot to achieve his dreams. He has secured the much-coveted Erasmus Mundus Joint Masters Scholarship with a whopping sum of € 33,600 for two years. With the 100% EMJM scholarship, he can now pursue QuanTEEM Master’s across four different universities, each semester in one of these universities:
SRM University-AP has numerous success stories and student accomplishments to share with the world. What makes the story of Bennet Benny different is the magnitude of his winning and the miles he has crossed after setting foot to achieve his dreams. He has secured the much-coveted Erasmus Mundus Joint Masters Scholarship with a whopping sum of € 33,600 for two years. With the 100% EMJM scholarship, he can now pursue QuanTEEM Master’s across four different universities, each semester in one of these universities:
University Bourgogne Franche-Comté (France)
Technische Universität Kaiserslautern (Germany)
Aarhus University (Denmark)
Moskovskiy Fiziko-Tekhnicheskiy Institut (Russia)
Internship at JAIST, Japan -2019
Like every other student, Bennet joined the Bachelor’s degree programme at the Department of Physics in 2018 with an irrepressible desire to dive into the depths of Physics. His undying passion for grasping the subject’s nuances is an influential lesson for all students to emulate. When he was in the second year of his graduate studies, Bennet won the Sakura Science Internship under the supervision of Prof. Ryo Maezono at JAIST, Japan. The internship was funded by the Japan Science and Technology Agency (JST), a government funding agency. For him, this was an excellent opportunity to learn more deeply about quantum mechanics. It also helped him raise his awareness of computational physics, its advantages, uses and the latest research around it.
“I was able to interact with many international scholars and researchers at the Japan Advanced Institute of Science and Technology. It helped me learn about the various ways through which I could fund my higher education. Therefore, after returning to SRM University-AP, I could work in the necessary direction to build my profile accordingly.” Bennet remarked. The enormous lessons he learned there helped him publish a research paper under the guidance of Prof Ranjit Thapa and his PhD students in the Computational Physics laboratory. “The Department of Physics has always motivated me to reach greater heights”, he added.
NTU-India Connect Research Programme 2022
Bennet has also proved his mettle by securing yet another internship opportunity as part of the NTU-India Connect Research Programme 2022. He was selected to spend a semester (Spring term) at NTU, Singapore with a full fellowship during the final year of graduation. As a young researcher, he has gained immense exposure and experience in such a short period giving him a competitive edge to move further in the direction of his dreams. “It has always been my dream to pursue a research career in Physics”, he asserted.
Looking Forward to QuanTEEM Master’s
The QuanTEEM Master’s programme is based on Quantum technologies and provides an excellent opportunity to build a research network in multiple countries in the European Union. He aspires to gain a deeper understanding of quantum mechanics and wishes to use the knowledge to improve our civilisation. In the words of Bennet, “We should never let the fear of failure deter us from trying. I feel that to reach our best potential; we need to face the challenges in life rather than be disheartened by them. Hence, I would encourage my fellow students never to hesitate to seek new opportunities.”
- Published in Academics, Blog, Departmental News, News, Physics News, Research, Sciences
Highly stable ruthenium catalyst for hydrogen evolution reaction
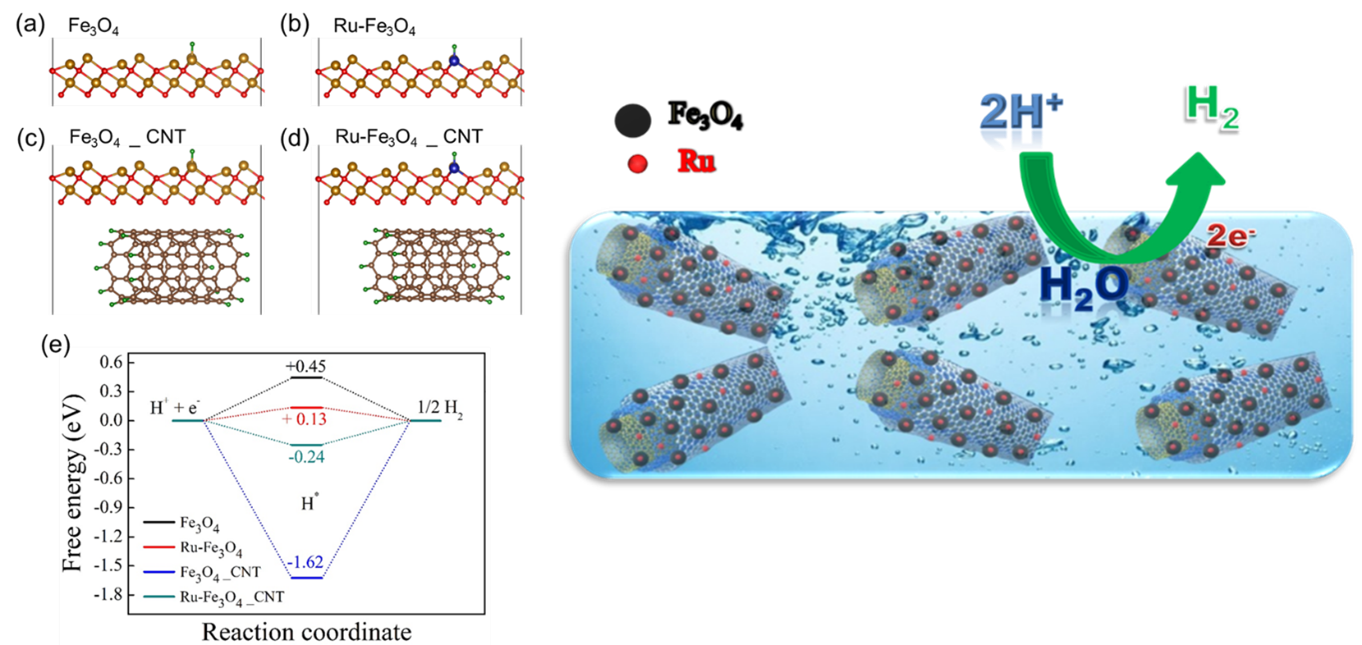
The Department of Physics is glad to announce that Prof Ranjit Thapa and his PhD scholar, Mr Samadhan Kapse, have published a patent titled “Highly Stable Ruthenium Single-Atom Catalysts on Fe3O4/MWCNTs for Hydrogen Evolution Reaction” (Application no. 202241006087). The research was done in collaboration with Ms Shwetha K R, Mr Shivanna M and Dr Nagaraju D H, from the Department of Chemistry, School of Applied Sciences, REVA University, Bangalore.
A Brief Description of the Research
In the current work, Fe3O4 nanoparticles were prepared by a simple chemical co-precipitation method under an inert atmosphere, and it was utilised for HER studies. Ru nanoparticles were profitably deposited over Fe3O4/MWCNTs modified glassy carbon electrode by the electrochemical deposition technique. The superior HER activity was achieved on Fe3O4/MWCNTs/Ru in 0.1M H2SO4 aqueous media. We demonstrated that synthesised electrocatalyst offers low over potential 101 mV to reach a current density of 10 mA cm-2 towards hydrogen evolution reaction. It displays exceptional stability and finds to be of no change in the HER activity despite 1000 cycles. It is emphasised that a small weight percentage of ruthenium in the prepared catalyst can replace high-cost platinum in renewable energy technologies.
Social Implications of the Research
Production of renewable energy has greater significance in the present situation owing to the impact of the depletion of non-renewable energy resources such as fossil fuels and the release of greenhouse gases into the atmosphere. Hydrogen has gained considerable interest as an energy storage and energy carrier due to its high energy density (146kJ/g), and its utilisation also eliminates pollution and toxicity. Several methods have been explored to produce molecular hydrogen. Among them, the electrolysis of water is the best way to produce high purity hydrogen from water. An excellent electrocatalyst is obligatory to liberate hydrogen gas effectively from water. It is known that Superior HER activity has been achieved using platinum (Pt) and Pt-based catalysts. Due to its high cost and low surplus, its expansion has been limited to the industrial scale. The research proposes that Ru-based catalysts can overcome these challenges.
DFT study is more effective to find the origin of catalytic activity in materials for designing highly promising catalysts for various catalytic reactions. The researchers expressed their gratitude to SRM University-AP for providing the required computational facility and support.
- Published in Departmental News, News, Physics News, Research News
DST-INSPIRE Subject Expert Committee Meeting
 A two-day DST-INSPIRE Subject Expert Committee meeting was held on July 14 & 15 at SRM University-AP campus. Experts in the area of Physical Sciences from across the country gathered at the university to evaluate this year’s INSPIRE Fellowship applications in Physical Sciences.
A two-day DST-INSPIRE Subject Expert Committee meeting was held on July 14 & 15 at SRM University-AP campus. Experts in the area of Physical Sciences from across the country gathered at the university to evaluate this year’s INSPIRE Fellowship applications in Physical Sciences.
INSPIRE Fellowship component offers 1000 Fellowships every year for carrying out doctoral degrees in both basic and applied sciences, including engineering and medicine, in the age group of 22-27 years.
The Chairperson of the Expert Committee in the area of Physical Sciences was Dr Dinakar Kanjilal, Professor, Inter-University Accelerator Centre (IUAC), New Delhi. “The fellowship ensures geographical distribution of excellence, and we look forward to more applicants from SRM AP”, Prof. Kanjilal said. Since its inception, SRM University-AP has INSPIRE Fellows as faculty members in the various departments of Sciences.
University Pro-Vice-Chancellor Prof D Narayana Rao, who also is the co-chair of the expert committee, said that it is gratifying to know that the PhD students have chosen to enrol in reputed universities and institutes. The applicants have chosen extremely accomplished scientists and faculty members as their research supervisors. “We are glad that 61 are girl students out of the 116 applications we received”, highlighted Prof D Narayana Rao. He expressed his happiness about the increasing number of women representation in Indian academia.
The other eminent scientists in the INSPIRE Fellowship selection committee included Member Secretary Dr Umesh K Sharma, Prof. Shikha Verma, Dr G. Vijaya Prakash, Dr AnandamayeeTej, Dr Rajendra Prasad Pant and Dr Arjit Chowdhuri.
Innovation in Science Pursuit for Inspired Research (INSPIRE)” is a flagship scheme of the Department of Science and Technology (DST), Government of India, which aims to attract meritorious youth to study basic and natural sciences at the college and university level and to pursue research careers in both basic and applied science areas including engineering, medicine, agriculture, and veterinary sciences.
- Published in Departmental News, News, Physics News, Research News
Published the first article under the Indo- Israel bilateral project
 Research at the Department of Physics envisions future studies on the anisotropic properties across various planes of their reported MAPbBr3 crystals and identifying the better plane for efficient electrical contact in device applications. Assistant Professor Dr Sabyasachi Mukhopadhyay and his research scholar Kunchanapalli Ramya recently published the paper Room-Temperature Cost-effective In-situ grown MAPbBr3 Crystals and their Characterization towards Optoelectronic Devices in the journal Material Science and Engineering: B. The paper has an impact factor of 3.407. They have done this work in collaboration with Sr Satyajit, IIT- Bhilai. This is the first article published under the Indo- Israel bilateral project A Halide Perovskite-Based Photoanode for Oxygen Evolution Reaction Using a Molecular Diode in a Hybrid Nanometer Scale.
Research at the Department of Physics envisions future studies on the anisotropic properties across various planes of their reported MAPbBr3 crystals and identifying the better plane for efficient electrical contact in device applications. Assistant Professor Dr Sabyasachi Mukhopadhyay and his research scholar Kunchanapalli Ramya recently published the paper Room-Temperature Cost-effective In-situ grown MAPbBr3 Crystals and their Characterization towards Optoelectronic Devices in the journal Material Science and Engineering: B. The paper has an impact factor of 3.407. They have done this work in collaboration with Sr Satyajit, IIT- Bhilai. This is the first article published under the Indo- Israel bilateral project A Halide Perovskite-Based Photoanode for Oxygen Evolution Reaction Using a Molecular Diode in a Hybrid Nanometer Scale.
Abstract
The paper reports the in-situ, room-temperature synthesis of methylammonium lead bromide CH3NH3PbBr3 crystals using N-methyl formamide as a source of methylammonium (MA+) ions during the crystallization process to explore the structural, dielectric, and electronic properties of CH3NH3PbBr3 crystals for optoelectronic applications. Optical absorption and radio-luminescence measurements affirm the direct bandgap nature of the crystals. Impedance spectroscopy measurements with various applied AC voltages within the 20 Hz – 10 MHz frequency range depict the influence of ionic motions on electrical transport across crystal planes. Researchers have extracted electrical transport parameters in CH3NH3PbBr3 crystals from the Nyquist plots, which we found to be distinctly varied wherein two different AC voltage amplitude regimes, broadly for 10 – 50 mV and 100 – 500 mV AC voltage range.
Explanation of the research
The wide approachability of our synthesis method lies in avoiding expensive precursor salts and eliminating the use of toxic solvents. We have obtained the MAPbBr3 crystals with improved thermal, optical, and dielectric properties that are used in optoelectronic devices, mainly in the applications of solar cells and photodetectors.
- Published in Departmental News, News, Physics News, Research News
Dr Jatis Kumar Dash to join the Editorial Board of Frontiers in Physics journal
 Frontiers in Physics is a peer-reviewed scientific journal. It covers the entire field of Physics ranging from experimental to computational and theoretical physics. Assistant Professor Dr Jatis Kumar Dash from the Department of Physics has been invited to be a part of the Editorial Board of this prestigious journal. The multidisciplinary journal focuses on applied physics and has an impact factor of 3.560.
Frontiers in Physics is a peer-reviewed scientific journal. It covers the entire field of Physics ranging from experimental to computational and theoretical physics. Assistant Professor Dr Jatis Kumar Dash from the Department of Physics has been invited to be a part of the Editorial Board of this prestigious journal. The multidisciplinary journal focuses on applied physics and has an impact factor of 3.560.
Dr Jatis Kumar Dash feels honoured to receive the invitation to join the Editorial Board. He finds it satisfying to get involved in scrutinising and reviewing manuscripts that concentrate on materials, experimental and condensed matter of Physics, and related devices. The process of reviewing these manuscripts enriches his knowledge in the subject domain. This helps him widen his research horison and explore novel ideas. The tenure of his role as an editor is not defined but is expected to last for five years.
- Published in Departmental News, News, Physics News
Exploring the charge transport across protein-based molecular junctions
 The latest research at the Department of Physics is investigating the charge transport across protein-based molecular junctions. Researchers envision fabricating bio- FETs which is useful in electronic devices as an alternative to Si- technology. Assistant Professor Dr Sabyasachi Mukhopadhyay and his PhD scholar Kunchanapalli Ramya published their paper Modulation of optoelectronic and mechanical properties across (bio) molecular junctions under external stimuli in the journal of Electronic Materials with an impact factor 2.04.
The latest research at the Department of Physics is investigating the charge transport across protein-based molecular junctions. Researchers envision fabricating bio- FETs which is useful in electronic devices as an alternative to Si- technology. Assistant Professor Dr Sabyasachi Mukhopadhyay and his PhD scholar Kunchanapalli Ramya published their paper Modulation of optoelectronic and mechanical properties across (bio) molecular junctions under external stimuli in the journal of Electronic Materials with an impact factor 2.04.
Abstract
Molecular junctions are formed by wedging molecules between two metal electrodes. Besides the conventional parameters of the metal-molecule-metal junction, such as the work function of electrodes and the molecules’ energy gap, molecule-electrode electronic coupling strength also plays a vital role in modulating the electronic properties of the molecular junction under external stimuli. We have also calculated several transport parameters which play a crucial role in finding the origin of conductance modulation under the external stimuli. We could find that before particular humidity conditions, the modulation in the conductance is due to the variation in coupling strength, which is due to the modulation in the electrostatic environment of retinal chromophores of protein by changing the structure of protein under various external stimuli.
Researchers have explored the external stimuli (illumination, force, and humidity conditions) effect on charge transport across bacteriorhodopsin-based molecular junctions. Their future research plans include bio- FET fabrication with the protein reported and studying the transistor characteristics across it.
- Published in Departmental News, News, Physics News, Research News
VS2-BP hybrid electrode material for supercapacitor applications
 A theoretical investigation is highly important to investigate the properties of materials, the origin of selectivity, and the effect of various parameters in designing promising electrode materials for supercapacitor applications. The latest research paper by Mr Samadhan Kapse, PhD Student in the Department of Physics, and Prof Ranjit Thapa, Associate Dean of SEAMS (Sciences), envisions this and developed a novel VS2-BP hybrid electrode material. Their article titled All-solid-state Supercapacitor Based on Advanced 2D Vanadium disulfide/Black Phosphorus Hybrids for Wearable Electronics has been published in the journal ACS Applied Energy Materials with an impact factor of 6.959.
A theoretical investigation is highly important to investigate the properties of materials, the origin of selectivity, and the effect of various parameters in designing promising electrode materials for supercapacitor applications. The latest research paper by Mr Samadhan Kapse, PhD Student in the Department of Physics, and Prof Ranjit Thapa, Associate Dean of SEAMS (Sciences), envisions this and developed a novel VS2-BP hybrid electrode material. Their article titled All-solid-state Supercapacitor Based on Advanced 2D Vanadium disulfide/Black Phosphorus Hybrids for Wearable Electronics has been published in the journal ACS Applied Energy Materials with an impact factor of 6.959.
Abstract
Vanadium disulfide-Black Phosphorus (VS2-BP) hybrids were synthesised by a one-pot hydrothermal assisted method to achieve enhanced electrochemical activity for supercapacitor applications. The concentration of BP was optimised to prevent the restacking nature of VS2 and to enrich the active edges for electrolytic ion intercalation. The charge storage kinetics of the best-performing VS2-BP as an active electrode has demonstrated the dominance of the pseudocapacitive nature of the material. Further, by sandwiching with PVA/K2SO4 gel electrolyte, an all-solid-state (ASS) Vanadium disulfide/Black Phosphorus-50 mg (VS2-BP-50) symmetric device was developed on highly conductive carbon paper. The ASS VS2-BP-50 symmetric device displays the highest specific areal capacitance of 203.25 mF/cm2. It exhibits the maximum areal energy density of 28.22 µW h cm-2 at an areal power density of 596.09 mW cm-2, outperforming previous literature. We used density functional theory to understand the origin of high quantum capacitance. We found that the charge accumulation region between VS2 and BP monolayers and the charge transfer is the origin of the improved density of states in the VS2-BP hybrid. Also, we observed the higher mobility of K+ ion and a higher diffusion rate using the Density functional theory (DFT) method.
Explanation of the research
A novel VS2-BP hybrid electrode material was prepared using a simple hydrothermal approach. Due to a synergistic effect, it was discovered that adding BP to metallic VS2 enhances the number of electrochemically active sites, resulting in increased surface activity. It also accelerates reaction kinetics with electrolyte ions by improving the electrical behaviour of active electrode material. As a result, the hybrid technique overcomes the weaknesses of individual components during electrochemical processes, resulting in increased performance that has been limited by individuals. The BP nanosheets behaved as a pore region for electron transport and prevented the VS2 layers from re-stacking. Systematic experiments are conducted by selecting the ideal precursor ratios to generate a high-quality VS2-BP hybrid with enhanced electronic conductivity. Furthermore, in the overall collective charge storage of the VS2-BP-50 hybrid material, the present results demonstrated that capacitive contributions outnumber diffusive contributions. The ASS VS2-BP-50 symmetric supercapacitor device was also designed to have a high areal capacitance of 203.25 mF/cm2 with a maximum areal power density of 596.09 mW cm-2. The extraordinary performance of the ASS VS2-BP-50 symmetric device illustrates its versatility in terms of designing a high-power density ASS supercapacitor for flexible and wearable device applications. The work functions of BP, VS2, and VS2-BP are 0.73 eV, 5.37 eV, and 4.99 eV, respectively, which help in the charge transfer mechanism and increase the density of state at the Fermi level, and subsequently, the quantum capacitance of the heterostructure.
Collaborations
1. Mr Aditya Sharma, Centre for Nano and Material Sciences, Jain Global Campus, Jakkasandra, Ramanagaram, Bangalore – 562112, Karnataka, India
2. Mr Ankur, Centre for Nano and Material Sciences, Jain Global Campus, Jakkasandra, Ramanagaram, Bangalore – 562112, Karnataka, India
3. Mr Sagar Bisoyi, Department of Physics, School of Applied Sciences, KIIT Deemed to be University, Bhubaneswar-751024, Odisha, India.
4. Dr Gopal K. Pradhan, Department of Physics, School of Applied Sciences, KIIT Deemed to be University, Bhubaneswar-751024, Odisha, India.
5. Dr Chandra Sekhar Rout, Centre for Nano and Material Sciences, Jain Global Campus, Jakkasandra, Ramanagaram, Bangalore – 562112, Karnataka, India
Social implications of the research
With the exponential development of portable/flexible electronics and the high demand for renewable energy, conventional energy-storage devices, such as supercapacitors, have attracted attention due to their benefits of fast charge/discharge rates, long cycle life, and high-power density. Similarly, developing novel functional materials with exceptional qualities could shed light on a plethora of challenges, including environmental pollution, energy crisis, etc. Two-dimensional (2D) layered materials, such as metallic 1T MoS2 single layers, SnSe2, MXenes, and black phosphorous (BP), have been intensively studied for supercapacitor applications. These materials benefit from efficient ion intercalation and electrosorption. The two-dimensional (2D) layered transition-metal dichalcogenides (TMDs) have recently piqued the scientific community’s curiosity.
- Published in Departmental News, News, Physics News, Research News
Efficient and selective single-atom catalysts for eNRR
The Department of Physics is glad to announce that Dr Ranjit Thapa and his PhD scholar Mr Samadhan Kapse have published their research paper “Descriptors and graphical construction for in silico design of efficient and selective single-atom catalysts for eNRR” in the journal Chemical Science, having an Impact Factor of 9.969. The paper was published in collaboration with Prof Shobhana Narasimhan, Theoretical Sciences Unit and School of Advanced Materials, Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore. Chemical Science is a highly prestigious nature Index journal, which accepts only breakthrough research contributions for publication.
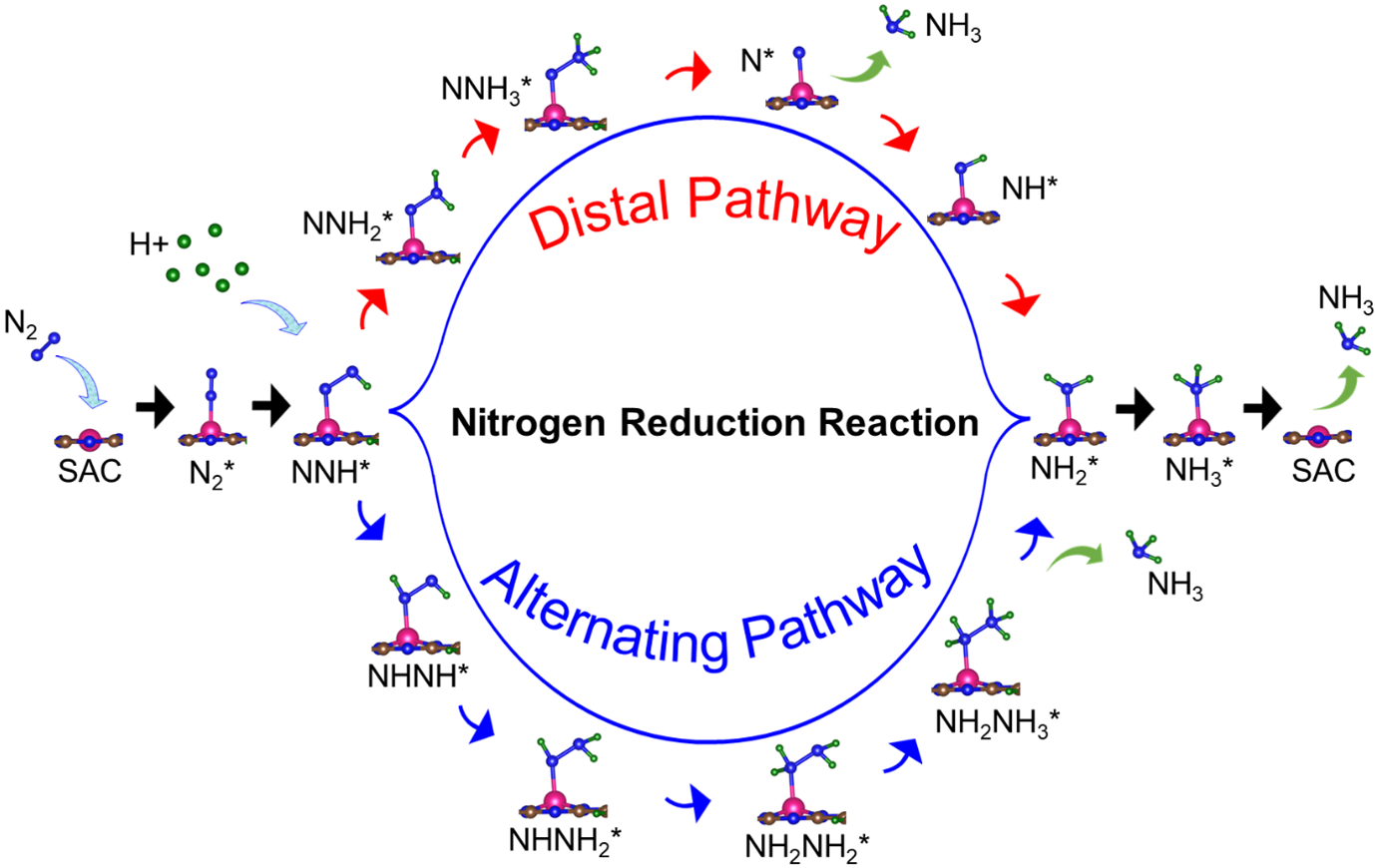
The Haber-Bosch process for ammonia synthesis has been described as possibly the most important scientific discovery of the twentieth century. However, it requires high temperatures and pressures and results in large energy consumption and emission of greenhouse gases. That is where electrochemical nitrogen reduction reaction (eNRR) comes into the picture. It synthesizes ammonia from nitrogen and water under mild conditions (N2 + 6H+ + 6e- → 2NH3). However, currently available eNRR catalysts need improvement in three respects: (i) the efficiency of nitrogen fixation needs to be increased, (ii) the competing hydrogen evolution reaction (HER) needs to be suppressed, and (iii) hydrogen poisoning of active sites must be avoided. Transition metals are popular eNRR catalysts; however, they tend to favour hydrogen adsorption due to the formation of strong metal d – hydrogen σ bonds, and tend to have a low affinity for N2 adsorption. Their research mitigates these problems by appropriately tuning the electronic structure by altering the environment surrounding metal atoms at the active site of single-atom catalysts (SACs). Moreover, in previous works, typically, only one criterion (usually competing HER) was used to optimize catalyst function, whereas they simultaneously optimised the catalyst function with respect to multiple criteria.
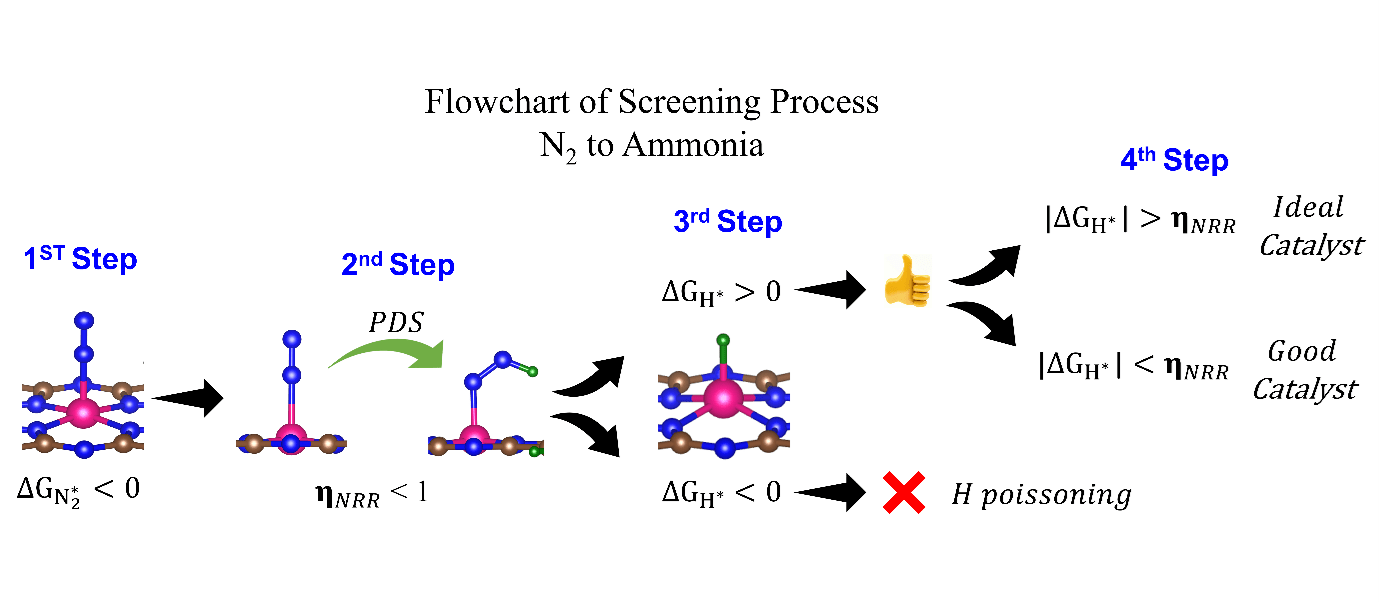
They have screened 66 different transition metal-based SACs for possible use in eNRR. To determine the best possible catalyst, they considered three factors: N2 adsorption, hydrogen poisoning and the overpotential of eNRR. Here, the valence electron occupancy (Oval) is identified as a new electronic descriptor that can predict the overpotential value. They emphasised that having a low η_NRR alone does not suffice to indicate a suitable eNRR catalyst, since if the adsorption free energy is higher for H than N2, active sites will be poisoned, hindering eNRR. Thus, they present a simple graphical procedure for identifying the most promising catalysts. To carry out this procedure, one must compute only 〖ΔG〗_(H^* ) and 〖ΔG〗_(NNH^* ), the changes in the free energies of H and NNH adsorption, respectively (note that η_NRR can be deduced if 〖ΔG〗_(NNH^* ) is known). The most promising candidate is identified as Sc-Pc, which they predict will have no H poisoning and will be highly selective for eNRR over HER. Moreover, they predict that Mn-Pc, Cr-N4, Fe-N2C2 should also be highly efficient, with low overpotential (η_NRR < 1 V) toward eNRR, and no H poisoning. In future they aim to find the selective materials for catalytic reactions by studying the origin of activity, reaction mechanism, etc.
Abstract of the Research
The electrochemical nitrogen reduction reaction (eNRR) offers the possibility of ammonia synthesis under mild conditions; however, it suffers from low yields, a competing hydrogen evolution reaction pathway, and hydrogen poisoning. We present a systematic approach toward screening single atom catalysts (SACs) for eNRR, by focusing on key parameters computed from density functional theory, and relationships between them. We illustrate this by application to 66 model catalysts of the types, TM-Pc, TM-NXCY, and TM-N3, where TM is a 3d transition metal or molybdenum. We identified the best SACs as Sc-Pc, Cr-N4, Mn-Pc, and Fe-N2C2; these show eNRR selectivity over HER and no hydrogen poisoning. The catalysts are identified through multi-parameter optimization which includes the condition of hydrogen poisoning. We propose a new electronic descriptor Oval, the valence electron occupancy of the metal center, that exhibits a volcano-type relationship with eNRR overpotential. Our multi-parameter optimization approach can be mapped onto a simple graphical construction to find the best catalyst for eNRR over HER and hydrogen poisoning.
- Published in Departmental News, News, Physics News, Research News
Large-scale production of BP nanosheets
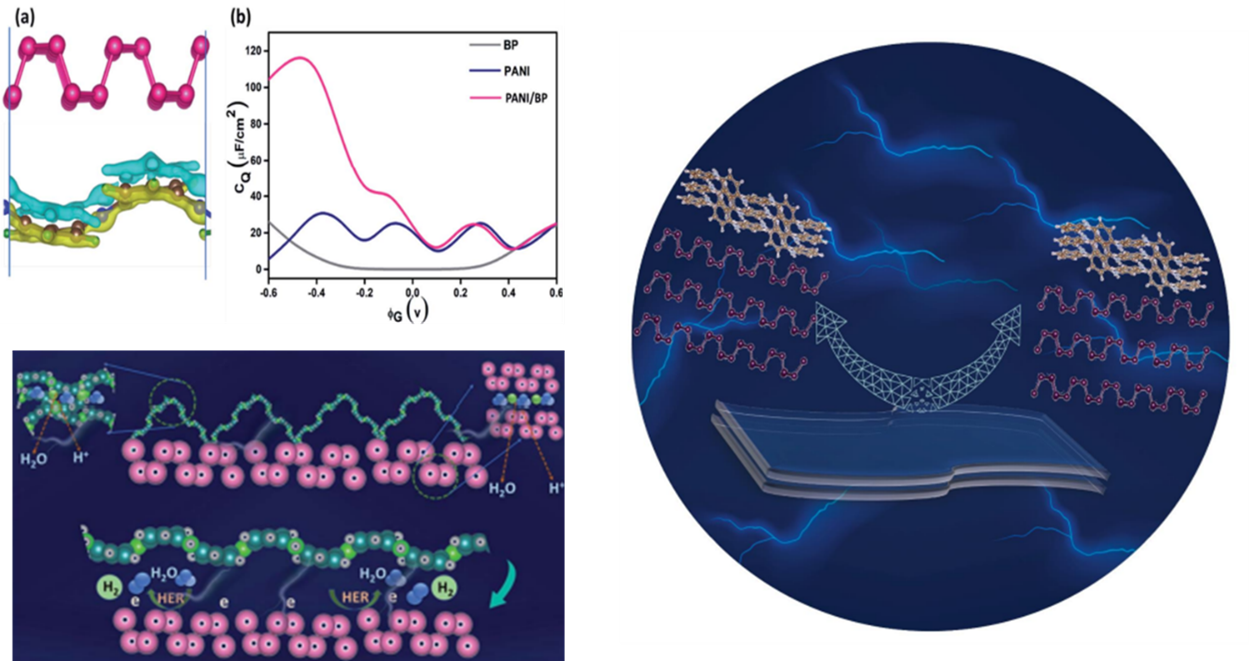 Research at the Department of Physics has effectively produced and characterised BP nanosheets on a large scale by a simple solvothermal approach, and the formation mechanisms are discussed. The paper, 2D-Black Phosphorus/Polyaniline Hybrids for Efficient Supercapacitor and Hydrogen Evolution Reaction Applications Check for updates, has been published by Prof Ranjit Thapa, Associate Dean of Sciences, as a corresponding author, and his PhD student, Mr Samadhan Kapse in Sustainable Energy & Fuels having an Impact Factor of 6.367.
Research at the Department of Physics has effectively produced and characterised BP nanosheets on a large scale by a simple solvothermal approach, and the formation mechanisms are discussed. The paper, 2D-Black Phosphorus/Polyaniline Hybrids for Efficient Supercapacitor and Hydrogen Evolution Reaction Applications Check for updates, has been published by Prof Ranjit Thapa, Associate Dean of Sciences, as a corresponding author, and his PhD student, Mr Samadhan Kapse in Sustainable Energy & Fuels having an Impact Factor of 6.367.
Abstract
Black phosphorous (BP) is an emerging 2D material with exciting physicochemical properties with broad applicability in electronics. Stability in the ambient environment, large-scale synthesis, and volume expansion during the charge/discharge process hinder its application in energy storage. Here, we report a facile gram-scale synthesis of BP in a mild reaction condition by a simple and cost-effective wet chemical method. To overcome its degradation and sluggish electrochemical performance, an organic hybrid with polyaniline is also prepared. Further, we fabricated a flexible supercapacitor device which results in an exceptional specific capacitance of 969 mFcm-2 at a current density of 0.4 Acm-2, which displayed a high energy density of 21.5 mWhkg-1 at a power density of 231 mWkg-1 with good cycling stability of 91% after 4000 charge-discharge cycles. Similarly, the cyclic voltammetry studies of the flexible devices at various bending angles display a similar CV profile for all the bending angles, which confirms the device’s reliability for flexible applications.
Explanation of the research
BP-PANI hybrid materials were prepared by the in-situ chemical oxidation method. By this approach, the researchers got highly stable BP by an inorganic-organic linkage, and its energy storage performance was also investigated. The fabricated symmetric flexible supercapacitor device based on BP/PANI heterostructure exhibited an extraordinary specific capacitance of 969 mFcm-2 at a current density of 0.4 Acm-2. Moreover, the fabricated device showed a high energy density of 21.5 mWhkg-1 and a power density of 231 mWkg-1 with impressive cycle stability of 91% after 4000 charge-discharge cycles. This study paves the way for future research into gram-scale BP synthesis, stability via an inorganic-organic coupling, and its potential application in electrochemical energy storage devices.
Social implications of the research
With the rapid growth of portable/flexible electronics and the high demand for clean energy, supercapacitors have sparked interest due to their advantages of fast charge/discharge rates, long cycle life, and high-power density compared to conventional energy-storage devices such as dielectric capacitors and Li-ion batteries. Likewise, developing new functional materials with outstanding properties could shed light on many issues, including pollution, energy, synthesis, and cost. In recent years few graphene analogues materials have been explored, and because of their tuneable physicochemical properties, they were used in energy storage applications. Generally, black phosphorus was synthesised from polymorphs of phosphorus under vigorous reaction conditions. However, these high temperature/pressure conditions suffer from safety, toxicity, controllability, and gram-scale production.
Quantum capacitance is an efficient tool for rapidly screening materials for supercapacitor applications and therefore is the future of this research. The researchers have collaborated with Mr Namsheer K, Mr Mridula Manoj, Mr Aditya Sharma, and Dr Chandra Sekhar Rout from the Functional Materials & Devices Laboratory, Centre for Nano Material Sciences, Jain University, Bangalore, India, in this work.
- Published in Departmental News, News, Physics News, Research News

